With the development of unshaped refractory castable,castable is more and more widely used in the lining of heating furnaces, especially low cement self-flow castable
Through reasonable selection of raw materials and particle size grading, with application of micro grade powder and high-efficiency dispersants, low cement self-flow castable is developed successfully. The self-flowing castable has the characteristics of convenient construction, self-flow and densification, good volume stability, good thermal shock stability, etc., and is suitable for parts with complex shapes and narrow spaces.
It is necessary to control the rheological properties, especially the setting time when low cement refractory castable is used in field. When the temperature in winter is lower than 5 °C, the hydration reaction is slower than that at normal temperature, and the hardening time of the castable is longer, the demolding time is greatly prolonged, which affects the subsequent performance of the castable and the construction progress.
In order to solve the problem of longer hardening time of low cement castables under low temperature such as winter, technicians used high alumina low cement self-flowing castables to study the effect of different accelerator types on the setting time to castables.
Theoretical support
CA and CA2 are the main hydraulic minerals of calcium aluminate cement, so the hydration and hardening of calcium aluminate cement are mainly the hydration of CA and CA2 and the crystal growth process of hydrate. The hydrates generated during the hydration of CA and CA2 vary with the curing conditions. At room temperature, CaO·Al2O3·10H2O (abbreviated as CA2H10), 2CaO·Al2O3·8H2O(C2AH8), and Al2O3·3H2O(AH3) are generated; In the hot and humid environment, both CAH10 and C2AH8 are rapidly converted into C3AH6. Among the hydration products of calcium aluminate cement, C3AH6 belongs to the cubic crystal system, and there are often many defects such as dislocations. Its crystal itself has low strength, combined with The strength is not high, so it does not contribute much to the strength of cement. CAH10 and C2AH8 belong to the hexagonal crystal system, and the crystals are in the form of flakes and needles, and are interlaced, overlapped and attached to each other to form a strong crystalline aggregate; the aluminum glue AH3 is filled in the gaps between the crystals to form a relatively dense structure, thus The cement obtains high mechanical strength. During the hydration process of calcium aluminate cement, it dissolves into water to release Ca2+ and Al(OH)4- ions. In order to improve the hydration and dissolution rate of cement, after adding Li+, Li+ in the solution forms insoluble hydroxides, such as lithium aluminum hydride (Li Al(OH)4), which reduces the Al(OH)4- ion concentration in the solution. It promotes the further dissolution of CA and CA2 in the cement, which is conducive to the formation of low-solubility calcium aluminate hydrate and promotes the deposition process.
Experimental results:
Using different coagulants (LiOH, NaOH, and sodium aluminate), in the same room temperature environment with the same amount of water (10%), the flow value and setting time of the castable samples after adding coagulants were observed.
Figure 1 shows the effect of low-cement self-flowing castables added with different accelerators on the flow properties. It can be seen that under the same amount of water (10%) and the indoor temperature of 19 °C, the flow value of the samples added with coagulants is reduced, and the most obvious is the addition of Li OH, which is different from coagulants. The rate of hydration of alkaline ions is related.

Figure 2 shows the setting time of each sample with the addition of different accelerators. From the perspective of setting time, the setting time of the sample added with LiOH is the fastest, and the setting time of the sample added with sodium aluminate and NaOH is not much different. The middle CA and CA2 were further dissolved, which accelerated the solidification time.
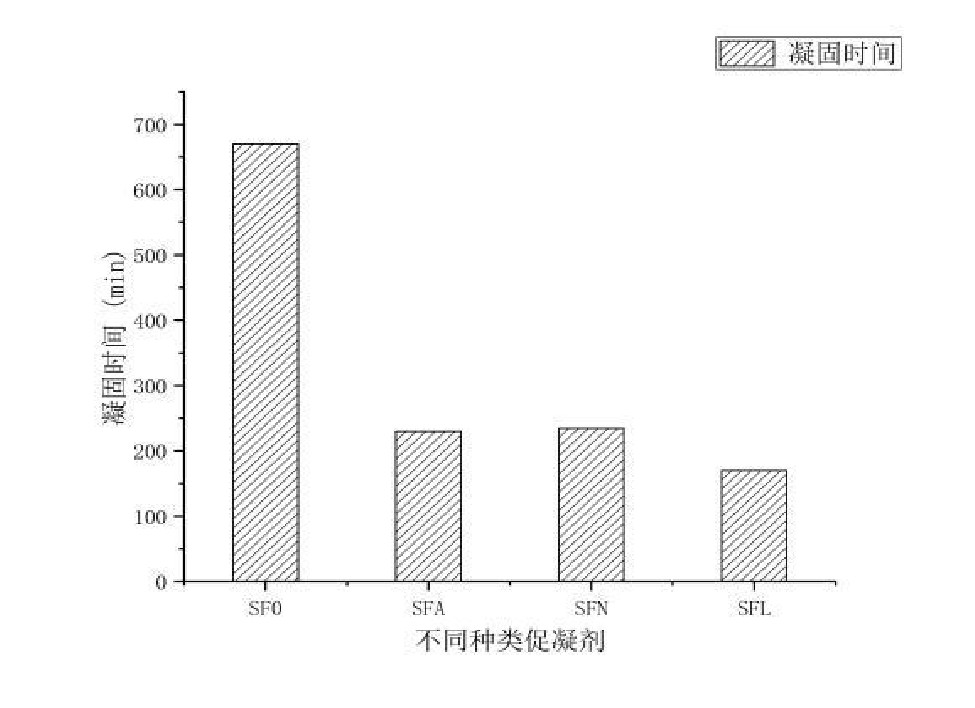
The results show that the addition of coagulant has little effect on the performance of low-cement self-flowing castables at room temperature, and the cold strength will be slightly improved. The setting time of the low-cement self-flowing castable was significantly reduced by adding the accelerator, and the setting time of the low-cement self-flowing castable was reduced from 11 h to 3 h after adding the accelerator. Among them, LiOH has the best coagulation effect, followed by sodium aluminate and NaOH. Adding a coagulant will slightly reduce the softening temperature under load of the sample, but it will not affect the normal use within the normal operating temperature range of the heating furnace.


